A method to expand LNP delivery capabilities to include small scaffold proteins such as nanobodies, DARPins, and Mini proteins.
Problem:
Most therapeutics require entry into the patient's cells to perform their intended actions, but the cellular membrane restricts what may enter the cell. Therefore, the therapeutics must be packaged into drug delivery systems to enter cells. Lipid nanoparticles (LNPs) are one such drug delivery method that has seen remarkable success in delivering DNA- and RNA-based therapeutics to cells. LNPs are safe, versatile, and highly efficient; however, LNPs have not worked well for delivering protein-based therapeutics, limiting their current utility.
Solution:
The inventors developed an LNP-based method to deliver small scaffold proteins to cells. They introduce a negatively charged protein tag to increase interactions between the protein and the LNPs.
Technology:
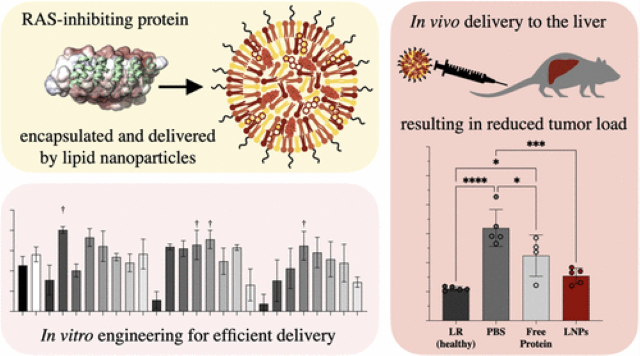
LNPs are fatty droplets that can pass through the cellular membrane. They work as a drug delivery mechanism by encapsulating the therapeutic and carrying it across the membrane. DNA and RNA are negatively charged and, therefore, easily interact with and become encapsulated by the positively charged LNPs. However, proteins are variably charged and therefore are more difficult to encapsulate in the LNPs. The inventors tag small scaffold proteins with negatively charged polypeptide tags to promote interactions between the protein and the LNPs. They find that this protein tag allows the proteins to become encapsulated by the LNPs and delivered into cells without compromising the protein's function.
Advantages:
- Allows for the LNP-based delivery of protein-based therapeutics (nanobodies, DARPins, and miniproteins).
- The presence of the anionic tag increases the number of cells receiving an anti-RAS DARPin (a cancer therapeutic) from 48% to 78% in a cell culture model.
- The presence of the anionic tag more than doubled the amount of anti-RAS DARPin delivered to each cell.
- The delivered anti-RAS DARPin reduced its target to 25% of the levels in control cells, indicating that the protein retains its therapeutic function.
Intellectual Property:
- Provisional Patent Application Filed
Case ID:
22-9996-tpNCS
Web Published:
10/30/2023
Patent Information:
| App Type |
Country |
Serial No. |
Patent No. |
File Date |
Issued Date |
Expire Date |